Oh or Cl Better Leaving Group
Weak bases have strong conjugate acids. The best leaving groups are WEAK BASES the worst leaving groups are.
Leaving Group Ability Is Increased By Acid Master Organic Chemistry
Iodide which is the least primary of the 4 frequent halides F Cl Br and I is the most effective leaving group amongst them.
. MOC members get access. Here is a wiki article fresh_42 on the leaving group. Oh shoot I forgot to post the link.
Oxygen As A Leaving Group Using Tosylate And Mesylate in Substitution and Elimination Reactions. Stability of leaving group LG The stability of leaving groups is another factor effecting the leaving group departure. Good leaving groups are weak bases.
What I dont get is that they then state I- is a better leaving group than Cl-. In this case I-. Oxygen makes a poor leaving group in substitution and elimination reactions.
What Makes a Good Leaving Group. Then in the leaving group section they say for the same types of reactions SN2 leaving group stability is inversely related to nucleophilicity and basicity and this makes sense as well because the less nucleophilic an ion the less it will resist being displaced. So it will not.
H2O is weak acid gives a stronger conjugate base OH-. When the leaving group leaves it takes its electrons back from the carbon atom to which they were shared during bond formation. An amide group b.
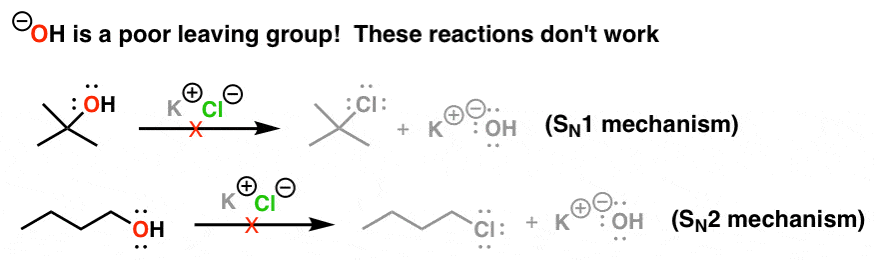
Fluoride is the least efficient leaving group among the many halides as a result of. Theyre happy and stable on their own. Think a strong base wants to react.
Cl- is a better leaving group than OH b. Cl- is a weak base therefore does not share electrons well d. Nucleophilicity is based on kinetics while basicity is based on thermodynamics.
Begingroup I think OH- is not a good leaving group as far as I know. So although the correlation is good its not perfect. So Cl-leaves faster than SH-.
Continue this thread level 1 5 yr. But leaving group ability is based on reaction rates. In general the weaker the base the better the leaving.
A leaving group LG is an atom or a group of atoms that is displaced as stable species taking with it the bonding electrons. 2 Convert the OH into a tosylate or mesylate using tosyl chloride or mesyl chloride. Application to SN1 and SN2 examples.
F- OH- OCH3- CN- N3- NH2- The best leaving groups are. Tosylate is often a better leaving group than halide as its preparation from an alcohol avoids stereochemical uncertainties and skeletal rearrangements. The better the leaving group the more likely it is to depart.
HCl strong acid lower pKa higher Ka so strong acid gives a weak conjugate base Cl-. EaRSH EaRCl EaRSH EaRCl. Nucleophilicy and basicity are two different terms.
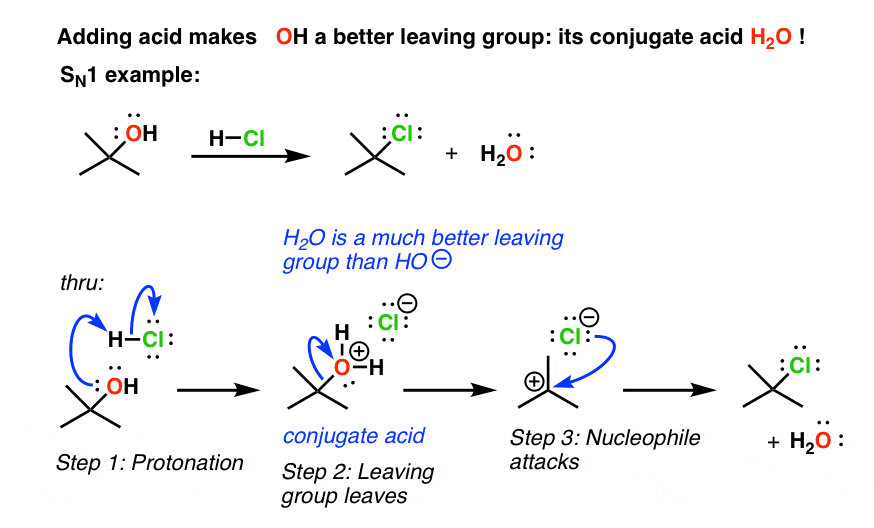
Reaction coordinate E Again lower electronegativity destabilizes both HS-and S in transition state. The product of these reactions is alkyl halide. That is the reason why we protonate it and then water is released as a good leaving groupStrong bases such as OH OR tend be poor leaving groups due their inability to stabilize a negative chargeFor a good leaving group the stabilization of charge is one of the factors.
Common anionic leaving groups are halides such as Cl Br. A nitrile group d. Add acid or convert to tosylatesmesylates.
A leaving group in my question deals with one that leaves with a charge or in the case with H2O and NH3 had a charge when attached but when it leaves the two groups have a zero charge. So we can identify weak bases by looking at a pK_a table. Weaker bases are higher leaving teams.
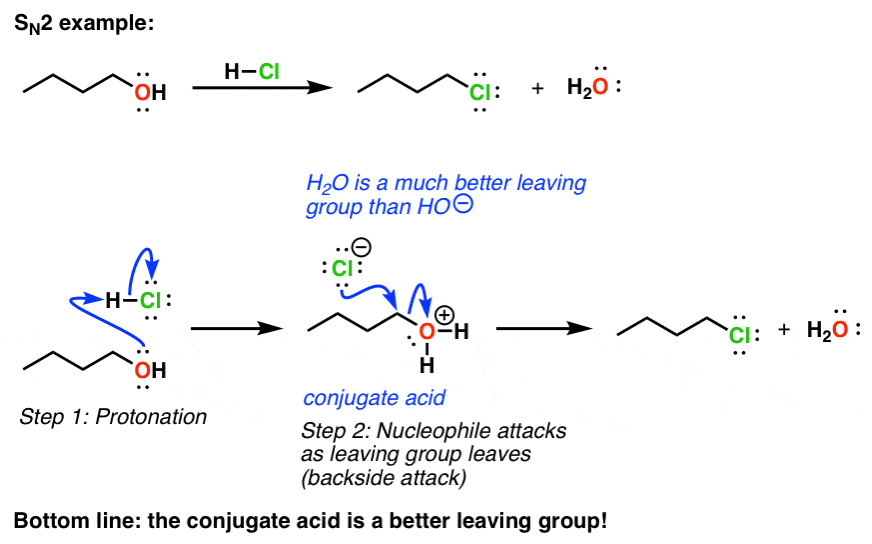
The SN2 reaction is bimolecular - it depends on amounts of nucleophile and leaving group SN2 reaction goes with inversion about the stereogenic carbon. H2O CH3OH -OTs -I Notes. This is why -OCH3 is a better leaving group but it does not imply anything about the nucleophilicity.
The pK_a value measures the position of an equilibrium. RH 2 C N N RH 2 C S C 4 F 9 O O RH 2 C S CH 3 O O RH 2 C I RH 2 C B r RH 2 C O H H RH 2 C O H CH 3 RH 2 C Cl RH 2 C N CH 3 CH 3 CH 3 D iazonium salt C lass of compound L eaving group Nonaf late C 4 F 9 SO 3-Mesylate Iodides B romides Protonated alcohols Protonated ethers Chlorides. For instance I- is a better nucleophile than F- because I- is a bigger molecule and it allows the electrons to be donated easily.
Good leaving groups are weak bases. Typically the leaving group is an anion eg. A nitro group c.
Common Leaving Groups RH 2 C N N Leaving group NC CH 2 R N N N. The delocalization of chlorines lone pair of electrons is minimal c. The reagents commonly used in substitution reactions are HBr HCl and HI.
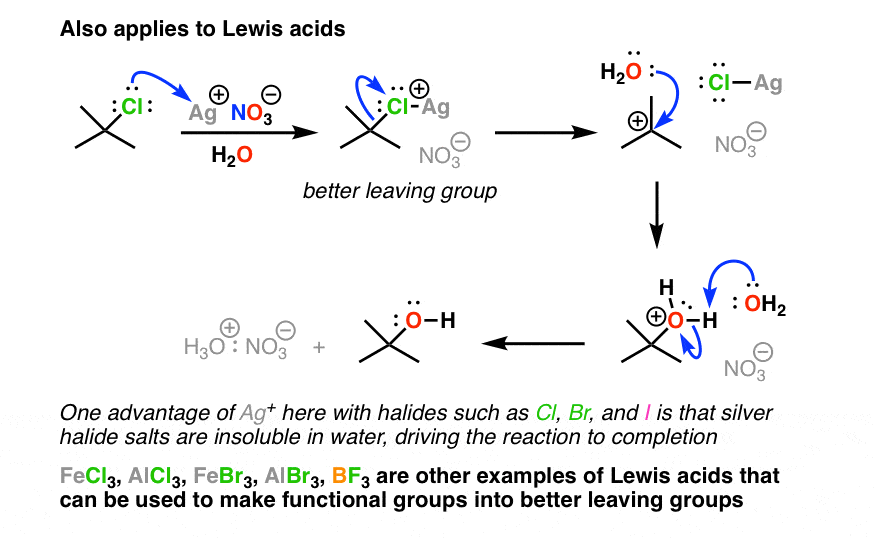
Strong base bad leaving group. Under acidic conditions the OMe group is protonated to make it a better leaving group so HOMe is the leaving group instead of OMe-. 1 Protonate the OH to give a water molecule which is an excellent leaving group.
2 key ways to make alcohols into good leaving groups. Halide ions I- Br- Cl- water OH2 and sulfonates such as p-toluenesulfonate OTs and methanesulfonate OMs. R-F R-Cl R-Br R-I.
Greater the stability of leaving group easier would be the leaving. In chemistry a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavageLeaving groups can be anions cations or neutral molecules but in either case it is crucial that the leaving group be able to stabilize the additional electron density that results from bond heterolysis. Under basic conditions OMe- generally doesnt leave but Grignard is such a strong nucleophile base that it forces the OMe- to.
All of the above Which of the following is formed when acetyl chloride reacts with an amine. OH Cl 3e Cl Cl 4e 31 8 OH 3f OTs 7 53 9 I 8 49 10 O 2N OH 3g O 2N Cl 4g 51 11 OCH 3 OH O 3h OCH 3 Cl O 4h 55 The pathway of the formation of the benzyl chorides is depicted in Scheme 2. For elimination reactions H 2 SO 4.
The key to kicking out an oxygen is to bribe it by turning it into a better leaving group. Because of TS stabilization not product stabilization What Makes a Good Leaving Group. Cl - or a neutral molecule eg.
Some examples of weak bases. Wants to take electrons electronegative. That is when left in its initial form.
Leaving Group Ability Is Increased By Acid Master Organic Chemistry
Leaving Group Ability Is Increased By Acid Master Organic Chemistry

Leaving Group Ability Is Increased By Acid Master Organic Chemistry
Comments
Post a Comment